Practice Guidelines
Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.
Evidence-Based Guidelines: Based on a systematic review, such that: 1). Clear description of search allows you to understand and even reproduce it. 2). Formal evaluation of the quality of included studies. 3). Recommendations based on higher quality studies and better evidence. 4). Explicit description of the strength of evidence for major recommendations.
Hybrid Guidelines: Begin with a recent evidence-based guideline, Review it and adapt to local circumstances, Avoid changing it too much! May improve buy-in and “ownership” of guideline
USPSTF: Established in 1984, makes recommendations on clinical preventive services offered or referred from the primary care setting: screening tests, counseling services, preventive medications. Recommendations apply to adults and children with no signs or symptoms: primary or secondary prevention. Currently 70 topics and about 200 recommendations. Independent panel of 16 unpaid experts in primary care medicine: family medicine (“GP”), pediatrics, obstetrics/gynecology, nursing. Carefully checked for financial conflict of interest. Serve 4 year terms as volunteers: 3 meetings per year + many phone calls + much reading and study.
USPSTF Process: Institute of Medicine recommends the USPSTF as a model for guideline development: Recommendations based on systematic reviews of the best available evidence. Considers benefits and harms, as well as certainty. Free of conflict of interest. Methods are transparent. Obtains public input and input from expert peer reviewers. Regularly updated (~ every 5 years).
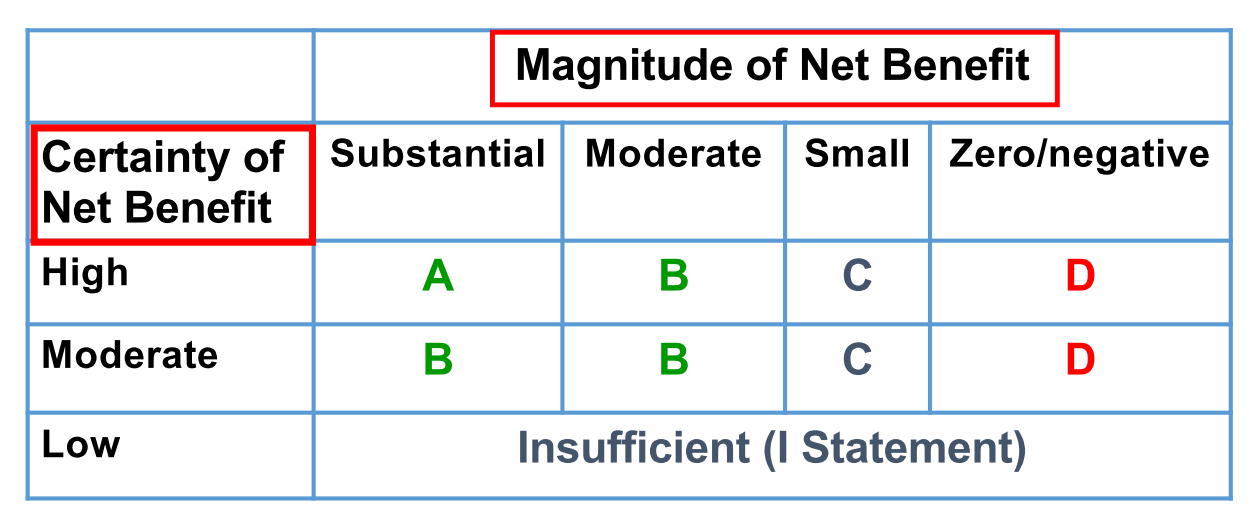
USPSTF steps: Step 1. Develop a Research Plan; Step 2. Develop a draft evidence report to answer each of the key questions. Step 3. Develop a draft recommendation. Focus is on net benefit (Net Benefit = Benefit - Harm). Step 4. Post draft recommendation for public comment. Step 5. Assign a grade to the recommendation. Step 6. Create Final Recommendation
Sources of Guidelines: National governments, States, Specialty societies (AAFP, ACP, ACOG, ACC, etc), Disease advocacy societies (American Cancer Society, American Heart Association), Hospitals and hospital system.
Evaluating the quality of practice guidelines
Comprehensive, Evidence-based, Practical/pragmatic/useful, Flexible, Information on benefits, harms and costs, Transparency and freedom from COI, Freely available, Regularly updated.
IOM Framework for Evaluating Guidelines: 1. Transparent process: the process for developing and funding the guideline should be clearly and transparently described. 2. Conflict of interest: none or few should have COI; chair or co-chair cannot have COI; financial ties that would create COI are eliminated. 3. Composition of guideline group: includes methods experts, clinicians, stakeholders, and patient representatives. 4. Systematic review: the guideline is based on the results of a good quality systematic review. 5. Strength of recommendation: this is clearly rated for each recommendation, using a taxonomy that incorporates strength of evidence and confidence in the recommendations. 6. Articulating recommendations: recommendations are clearly and concisely listed, and are actionable. 7. External review: stakeholders, experts, and others provide external peer review of the guidelines, including opportunity for public comment. 8. Updating: A process for updating the guideline is stated.